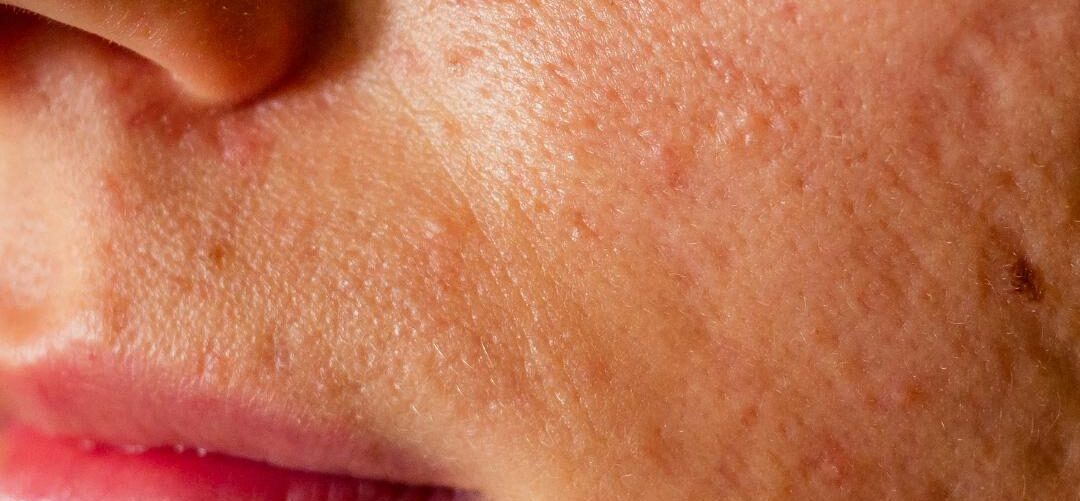
Atrophic Scars
 Faramarz Rafie MD / Vancoderm Academy and College [VDA] / Vancoderm Clinic [VDCMed]
Faramarz Rafie MD / Vancoderm Academy and College [VDA] / Vancoderm Clinic [VDCMed]
Atrophic scars represent one of the most prevalent and psychologically distressing long-term sequelae of inflammatory and traumatic dermal injury, with acne vulgaris accounting for the majority of cases. From a medical standpoint, atrophic scarring is not merely a cosmetic concern but a structural failure of dermal repair, resulting in permanent alterations to skin architecture.
Unlike hypertrophic or keloid scars—which arise from excessive fibroblast activity and collagen overproduction—atrophic scars are defined by a net loss of dermal tissue, specifically collagen, elastin, and extracellular matrix components. This deficit produces visible depressions below the level of the surrounding epidermis, often accompanied by irregular texture, shadowing, and altered light reflection, which magnify their clinical appearance.
Clinical and Biological Basis
Atrophic scars develop when the degree, duration, and intensity of inflammatory tissue destruction exceed the skin’s intrinsic regenerative capacity during wound healing. In normal cutaneous repair, inflammation is a controlled, time-limited phase that initiates fibroblast recruitment, angiogenesis, and extracellular matrix (ECM) reconstruction. In atrophic scarring, this balance is disrupted, leading to net tissue loss rather than restoration.
Inflammatory Cascade and Dermal Damage
In acne vulgaris, inflammation originates within the pilosebaceous unit, where follicular obstruction, Cutibacterium acnes proliferation, and rupture of the follicular wall expose the dermis to keratin, sebum, and bacterial antigens. This triggers an exaggerated innate immune response characterized by:
- Release of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α)
- Activation of neutrophils and macrophages
- Sustained oxidative stress
- Prolonged enzymatic tissue degradation
- Rather than resolving efficiently, chronic inflammation persists, causing progressive dermal injury.
Role of Matrix Metalloproteinases (MMPs)
A central mechanism in atrophic scar formation is the upregulation of matrix metalloproteinases, particularly MMP-1, MMP-3, and MMP-9. These zinc-dependent enzymes are responsible for collagen breakdown during normal tissue remodeling. However, in chronic inflammatory states:
- MMP activity exceeds collagen synthesis
- Type I and III collagen fibers are prematurely degraded
- The extracellular matrix scaffold collapses
- Simultaneously, tissue inhibitors of metalloproteinases (TIMPs), which normally regulate MMP activity, are downregulated, allowing unchecked matrix destruction.
Fibroblast Dysfunction and Apoptosis
- Fibroblasts are the primary cells responsible for collagen synthesis, elastin production, and ECM maintenance. In atrophic scarring:
- Pro-inflammatory cytokines induce fibroblast apoptosis
- Remaining fibroblasts exhibit reduced proliferative capacity
- Transforming growth factor-β (TGF-β) signaling is impaired
- Collagen gene expression (COL1A1, COL3A1) is suppressed
- As a result, collagen replacement during the remodeling phase is insufficient, delayed, and disorganized.
Vascular Compromise and Hypoxia
Sustained inflammation also disrupts dermal microvasculature:
- Capillary damage reduces oxygen and nutrient delivery
- Impaired angiogenesis limits tissue regeneration
- Chronic hypoxia further suppresses fibroblast activity
- This vascular insufficiency contributes to poor-quality scar tissue and failure of normal dermal thickening.
Defective Remodeling Phase
- The remodeling phase of wound healing, which may last months to years, is responsible for replacing provisional collagen with mature, well-organized fibers. In atrophic scars:
- Collagen remodeling is incomplete
- Collagen fibers remain thin, fragmented, and misaligned
- Dermal thickness fails to recover to baseline
- Mechanical strength of the tissue is reduced
- Instead of restoring normal architecture, the dermis remains structurally deficient and permanently depressed.
Histopathological Characteristics
From a microscopic perspective, atrophic scars exhibit several defining features:
Thinning of the Reticular Dermis
The reticular dermis, which provides tensile strength and volume, is significantly reduced in thickness due to collagen loss and impaired regeneration.
Disorganized and Fragmented Collagen Bundles
Collagen fibers appear:
- Sparse
- Irregularly oriented
- Loosely packed
This contrasts sharply with the dense, parallel collagen bundles seen in normal dermis.
Reduced Fibroblast Density
There is a measurable reduction in fibroblast number and activity, limiting the tissue’s ability to remodel and repair.
Loss of Elastin Fibers
Elastin degradation leads to:
- Reduced recoil
- Poor skin resilience
- Increased visibility of surface depressions
Fibrotic Tethering to Deeper Structures
In rolling scars, fibrous bands extend from the dermis into the subcutaneous tissue, anchoring the skin downward. These adhesions prevent surface elevation even when collagen is partially restored.
Practical Clinical Classification Clinically, atrophic scars are classified based on morphology, depth, and tethering, as these factors directly determine treatment selection:
Ice Pick Scars
- Narrow (<2 mm), deep, sharply marginated
- Extend into deep dermis or subcutaneous tissue
- Poor response to resurfacing alone
- Require focal surgical or chemical reconstruction
Boxcar Scars
- Wider, U-shaped depressions with well-defined edges
- Can be shallow or deep
- Respond variably to resurfacing and collagen induction
Rolling Scars
- Broad, undulating depressions
- Caused by fibrous septae anchoring the dermis to deeper tissue
- Best treated with subcision and collagen stimulation
Medical Implications for Treatment Planning
Management of atrophic scars requires interventions that target the underlying dermal pathology—fibrotic tethering, collagen and elastin deficiency, and tissue volume loss.
Subcision is a cornerstone mechanical procedure designed to release fibrotic bands tethering the dermis to deeper structures. By physically severing these adhesions, subcision converts chronically depressed scar tissue into an acute wound environment, stimulating localized inflammation, fibroblast activation, and neocollagenesis. Subcision is often combined with adjunctive therapies such as platelet-rich plasma (PRP) or injectable fillers to prevent re-tethering and enhance the regenerative milieu.
Collagen induction therapies aim to restore dermal integrity through controlled injury. Microneedling, including radiofrequency-assisted Microneedling, creates microchannels in the dermis, stimulating fibroblast proliferation, upregulation of type I and III collagen synthesis, and elastin regeneration. Fractional laser therapy employs ablative or non-ablative microthermal zones to selectively injure the dermis, prompting remodeling while sparing surrounding tissue. Both approaches require multiple treatment sessions over weeks to months to achieve measurable increases in dermal thickness and improvement in scar morphology.
Volume restoration addresses the actual loss of dermal and subcutaneous tissue inherent to deep boxcar and rolling scars. Hyaluronic acid fillers provide immediate structural correction by mechanically elevating the scar base, restoring skin contour, and improving light reflection. Biostimulatory injectables, such as poly-L-lactic acid or calcium hydroxylapatite, induce long-term collagen deposition, gradually increasing dermal volume and resilience. Strategic application ensures both immediate and sustained improvement while complementing collagen induction procedures.
Chemical reconstruction, most notably the TCA CROSS (trichloroacetic acid chemical reconstruction of skin scars) technique, is particularly effective for narrow, deep ice pick scars. High-concentration TCA is applied focally to the base of the scar, inducing controlled chemical injury that triggers localized collagen synthesis and gradual elevation of the depressed tissue. This method allows precise remodeling of scars that are typically resistant to conventional resurfacing techniques.
Collectively, these modalities are frequently employed in combination protocols, allowing a comprehensive approach that addresses mechanical tethering, dermal regeneration, and volume restoration, which is essential for optimal and durable correction of atrophic scars.
Studies demonstrate significant associations with reduced self-esteem, social anxiety, and depression, reinforcing the need for early intervention and realistic patient counseling.
 Vancoderm Academy and College stands at the forefront of academic education in medical aesthetics, offering the Clinical Practitioner Specialist Diploma in Medical Aesthetics, a comprehensive program designed to equip students with advanced clinical knowledge and hands-on expertise in skin, laser, and aesthetic therapies. Our next intake is scheduled for February 1, 2026, and we invite aspiring practitioners to join our growing community of skilled professionals. We sincerely thank our readers for their continued support and engagement, and encourage you to follow us on Instagram, Facebook, and LinkedIn for updates, insights, and educational resources. Wishing all our students and followers the very best in their professional journey.
Vancoderm Academy and College stands at the forefront of academic education in medical aesthetics, offering the Clinical Practitioner Specialist Diploma in Medical Aesthetics, a comprehensive program designed to equip students with advanced clinical knowledge and hands-on expertise in skin, laser, and aesthetic therapies. Our next intake is scheduled for February 1, 2026, and we invite aspiring practitioners to join our growing community of skilled professionals. We sincerely thank our readers for their continued support and engagement, and encourage you to follow us on Instagram, Facebook, and LinkedIn for updates, insights, and educational resources. Wishing all our students and followers the very best in their professional journey.
Thank you!