Exosomes in Aesthetic Medicine: A New Frontier in Skin Regeneration
Faramarz Rafie MD, /Vancoderm Academy [VDA]/ Vancoderm Clinic [VDCmed]
 In the ever-advancing world of aesthetic medicine, few innovations have generated as much excitement as exosome therapy. These microscopic biological messengers, once known only in advanced biomedical research, are now making their way into skin clinics and regenerative treatment rooms worldwide. Unlike traditional skincare ingredients that act superficially, exosomes work at the cellular communication level — delivering signals that tell skin cells how to repair, regenerate, and restore balance.
In the ever-advancing world of aesthetic medicine, few innovations have generated as much excitement as exosome therapy. These microscopic biological messengers, once known only in advanced biomedical research, are now making their way into skin clinics and regenerative treatment rooms worldwide. Unlike traditional skincare ingredients that act superficially, exosomes work at the cellular communication level — delivering signals that tell skin cells how to repair, regenerate, and restore balance.
For aesthetic practitioners, this means a new tool that can accelerate recovery after procedures, enhance collagen remodeling, improve skin texture, and even stimulate hair growth — all while supporting the body’s own repair mechanisms. Whether used on their own or paired with advanced delivery systems like Microneedling, exosomes are shaping the future of skin rejuvenation with results that are both science-driven and clinically impressive.
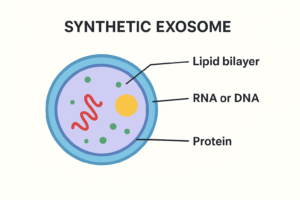
Exosomes are nano-sized extracellular vesicles (30–150 nanometers in diameter) secreted by nearly all cell types, particularly mesenchymal stem cells, fibroblasts, and immune cells. They are enclosed by a lipid bilayer membrane and contain a complex cargo of proteins, lipids, messenger RNA (mRNA), microRNA (miRNA), and other bioactive molecules.
Functionally, exosomes serve as intercellular communication vehicles, transferring their molecular contents from one cell to another. This exchange can modulate gene expression, regulate inflammatory pathways, stimulate tissue repair, and influence cellular differentiation.
In the context of aesthetic medicine, exosomes derived from regenerative cell sources (such as mesenchymal stem cells) are leveraged for their ability to:
- Promote fibroblast proliferation and collagen synthesis
- Enhance angiogenesis and microcirculation
- Modulate inflammation to accelerate post-procedure healing
- Support skin barrier restoration and pigmentation balance
Because exosomes are cell-free biologics, they carry regenerative signals without introducing live cells, which enhances their safety profile while maintaining potent therapeutic potential.
Secretome Versus Exosome:
The Secretome refers to the complete set of bioactive molecules that a cell secretes into its surrounding environment. It represents the cell’s way of communicating and influencing neighboring or distant cells. The secretome is not limited to one type of molecule; rather, it is a complex mixture that includes soluble proteins such as growth factors, cytokines, chemokines, and enzymes, as well as lipids, nucleic acids, and extracellular vesicles like exosomes and microvesicles. Because of this diversity, the secretome plays a central role in regulating a wide range of biological processes, including inflammation, angiogenesis, immune modulation, and tissue repair. For example, the secretome of mesenchymal stem cells (MSCs) contains vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), interleukin-10 (IL-10), and exosomes, all of which work synergistically to promote wound healing, regeneration, and anti-inflammatory responses. Unlike single-molecule therapies, the secretome offers a cocktail of factors that can act together, making it highly valuable in regenerative medicine, dermatology, and drug development.
As discussed earlier, the exosome is a nanosized extracellular vesicle (30–150 nm) that represents a specialized component of the secretome. Unlike soluble molecules that freely diffuse and may degrade quickly, exosomes are enclosed by a lipid bilayer membrane, which protects their cargo and allows for stable transport through bodily fluids. They carry a selective and biologically active payload, including proteins, lipids, DNA, mRNA, and microRNAs, which are delivered directly into recipient cells to modify their behavior. Exosomes play a vital role in intercellular communication by transferring these functional molecules with high specificity, often influencing processes such as cell proliferation, immune regulation, angiogenesis, and tissue repair. For instance, mesenchymal stem cell–derived exosomes are rich in microRNAs and growth factors that enhance fibroblast activity, stimulate collagen synthesis, reduce inflammation, and accelerate wound healing. Because of their stability, biocompatibility, and natural targeting capabilities, exosomes are increasingly being explored as therapeutic agents and drug delivery vehicles in regenerative medicine, oncology, neurology, dermatology and Aesthetic Medicine.
Variations in Exosome Preparations, Not all exosome products are identical.
Natural Exosomes: Key variations include:
- Source
- Mesenchymal Stem Cell (MSC)-Derived – Most common in aesthetics, known for regenerative and anti-inflammatory properties.
- Platelet-Derived – Sometimes combined with PRP to enhance tissue healing.
- Fibroblast-Derived – Used for targeted collagen remodeling.
- Formulation
- Pure Isolated Exosomes – Freeze-dried or in liquid suspension.
- Exosome-Enriched Conditioned Media – Contains both exosomes and other growth factors.
- Additives
- Some formulations include vitamins, peptides, or hyaluronic acid for synergistic effects.
Synthetic Exosomes:
Synthetic exosomes are artificially engineered vesicles designed to mimic the structure and function of natural exosomes while overcoming some of their limitations. Natural exosomes are highly effective carriers for drug delivery and cell communication, but their clinical use is hindered by challenges such as heterogeneity, difficulty in large-scale isolation, and batch-to-batch variability. To address these issues, researchers have developed synthetic or bioinspired exosomes that can be customized, standardized, and mass-produced for therapeutic applications.
One approach to creating synthetic exosomes is top-down engineering, where natural cell membranes are broken down and reassembled into vesicles that resemble exosomes. This maintains some of the natural surface proteins responsible for targeting, while allowing better control over vesicle size and content. Another approach is bottom-up synthesis, which uses defined lipids, proteins, and polymers to build exosome-mimetic nanoparticles from scratch. This method allows for precise control of composition, surface modifications, and drug loading, making it easier to tailor the vesicles for specific therapeutic needs.
Synthetic exosomes have multiple potential applications. In drug delivery, they can be loaded with chemotherapeutics, nucleic acids (siRNA, miRNA, mRNA, CRISPR components), or small molecules, and surface-engineered with ligands to target specific tissues or tumor cells. In gene therapy, synthetic exosomes provide a safer alternative to viral vectors, with less risk of immune reactions or insertional mutagenesis. They are also being investigated in neurological diseases, since they can be designed to cross the blood–brain barrier, and in regenerative medicine, where they can carry growth factors or signaling molecules to promote tissue repair. Furthermore, synthetic exosomes are explored as vaccine delivery systems, carrying antigens or mRNA to stimulate targeted immune responses.
The advantages of synthetic exosomes include scalability, reproducibility, safety, and customizability. Unlike natural exosomes, which vary depending on their cellular source, synthetic versions can be standardized for clinical-grade manufacturing. They also reduce the risk of transferring unwanted biological material, such as oncogenic proteins or viral components. However, challenges remain, including replicating the full complexity of natural exosome functions, ensuring long-term stability in circulation, and validating their safety in large-scale clinical trials.
different sources of exosomes have been studied for specific effects on fibroblast activity, pigmentation, and skin healing. Exosomes are not all the same; their cargo depends on the cell of origin, which makes some more suitable for particular dermatological or aesthetic purposes.
Exosomes for Fibroblast Stimulation & Collagen Production
- Mesenchymal Stem Cell (MSC)–derived exosomes are the most widely studied for skin rejuvenation.
- They stimulate fibroblast proliferation, migration, and collagen synthesis.
- They carry miRNAs and growth factors that upregulate extracellular matrix production.
- Benefits: improved skin elasticity, reduced wrinkles, and enhanced dermal regeneration.
Exosomes for Pigmentation Regulation
- Adipose-derived stem cell exosomes (ADSC-Exos) have shown activity in reducing melanogenesis by downregulating MITF and tyrosinase (key pigmentation regulators).
- This results in skin brightening and reduction of hyperpigmentation.
- Keratinocyte-derived exosomes may also influence melanocytes, either stimulating or inhibiting pigmentation depending on their state.
- Clinical potential: adjunct in treating melasma, post-inflammatory hyperpigmentation, and uneven skin tone.
Exosomes for Skin Healing & Repair
- MSC-Exos accelerate wound healing by promoting angiogenesis, keratinocyte migration, and anti-inflammatory responses.
- Fibroblast-derived exosomes can directly enhance dermal repair, increasing matrix remodeling.
- Platelet-derived exosomes are rich in growth factors (VEGF, PDGF, TGF-β), supporting tissue regeneration and faster healing.
- Applications: post-laser recovery, scar reduction, chronic wound healing, and post-inflammatory repair.
Mechanism of Action in Skin Regeneration
When applied to skin, exosomes:
- Bind to Cell Receptors – Triggering signaling pathways in target cells.
- Deliver Bioactive Cargo – Proteins and genetic material alter cell function to stimulate repair.
- Promote Fibroblast Activity – Boosting collagen, elastin, and hyaluronic acid synthesis.
- Reduce Inflammation – Modulating immune responses to minimize redness and downtime.
- Enhance Angiogenesis – Supporting microcirculation for better nutrient and oxygen supply.
 Exosome applications in drug delivery systems
Exosome applications in drug delivery systems
The result is improved texture, elasticity, hydration, and overall skin health.
Exosomes are nanosized extracellular vesicles secreted by almost all cell types, and they play a key role in intercellular communication by transporting proteins, lipids, and nucleic acids. Their biocompatibility, stability in circulation, and natural ability to cross biological barriers, such as the blood–brain barrier, make them highly attractive as natural drug delivery vehicles. Unlike synthetic nanoparticles, exosomes are derived from biological systems, which reduces toxicity and immune rejection while improving the efficiency of drug delivery.
One of the most promising applications of exosomes is in cancer therapy. They can be engineered to carry chemotherapeutic agents such as paclitaxel or doxorubicin, thereby enhancing targeted drug delivery to tumors while minimizing systemic toxicity. Exosomes can also deliver genetic materials, including siRNA, miRNA, and CRISPR/Cas9 components, to silence oncogenes or modulate signaling pathways involved in tumor progression. Additionally, immune cell–derived exosomes, such as those from dendritic cells or natural killer cells, can stimulate anti-tumor immune responses, providing a dual therapeutic effect.
In neurological disorders, exosomes have shown particular value because they can cross the blood–brain barrier, which poses a major obstacle for conventional drug delivery systems. They have been explored as carriers for drugs, anti-inflammatory molecules, and nucleic acids in the treatment of Alzheimer’s disease, Parkinson’s disease, brain tumors, and stroke. This makes them a promising strategy for central nervous system–targeted therapies. Similarly, in gene therapy, exosomes can deliver therapeutic nucleic acids without the risks associated with viral vectors. Engineered exosomes carrying mRNA, siRNA, or gene-editing tools offer a safe and efficient platform for correcting genetic disorders or regulating gene expression.
Exosomes also hold great promise in regenerative medicine. Those derived from mesenchymal stem cells are rich in growth factors, cytokines, and regulatory miRNAs, which promote tissue repair, angiogenesis, and wound healing. This has opened possibilities for their use in cardiac repair following myocardial infarction, liver regeneration, and skin rejuvenation. Beyond regenerative applications, exosomes are being studied for their role in treating autoimmune and inflammatory diseases. By loading them with anti-inflammatory drugs such as curcumin or dexamethasone, or immunomodulatory molecules, they can be directed to inflamed tissues, offering new treatment avenues for conditions like rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis.
In infectious diseases and vaccine development, exosomes are being harnessed as carriers of antigenic proteins or nucleic acids to elicit robust immune responses. For example, exosome-based platforms are under investigation for developing vaccines against viruses such as HIV and SARS-CoV-2. Their natural role in antigen presentation further enhances their effectiveness as vaccine carriers.
Overall, exosomes present several advantages over conventional delivery systems, including excellent biocompatibility, inherent targeting capabilities, and the ability to protect therapeutic cargo from degradation. However, challenges such as large-scale isolation, purification, heterogeneity of exosome populations, and concerns about long-term safety must be addressed before their widespread clinical adoption. Despite these obstacles, exosomes remain one of the most promising next-generation platforms for drug delivery, with applications spanning oncology, neurology, gene therapy, regenerative medicine, and immunotherapy.
Indications in Aesthetic Medicine
Exosome therapy is used for:
- Skin Rejuvenation – Fine lines, wrinkles, dullness.
- Acne Scarring – Promoting dermal remodeling.
- Hyperpigmentation – Regulating melanocyte activity.
- Post-Laser or RF Recovery – Accelerating healing and reducing downtime.
- Hair Restoration – Supporting follicle growth and density.
- Skin Barrier Repair – For sensitive, inflamed, or compromised skin.
Contraindications
Avoid or postpone exosome therapy in:
- Active skin infections or herpes simplex outbreaks.
- Active inflammatory skin diseases (e.g., psoriasis flare).
- Known allergy to any product components.
- Pregnancy or breastfeeding (lack of safety data).
- Patients with active cancer (until cleared by physician).
Possible Side Effects and Adverse Effects
Generally well tolerated, but potential reactions may include:
- Temporary redness, swelling, or warmth at the application site.
- Mild itching or dryness for 24–48 hours.
- Rare hypersensitivity or allergic reaction.
Using Exosomes in Aesthetic Practice
Exosomes can be integrated into various treatment modalities:
- Microneedling / Dermal Pen Infusion
- Fractional Laser Post-Treatment
- Radiofrequency Microneedling (RF-MN)
- Mesotherapy or Electroporation Devices
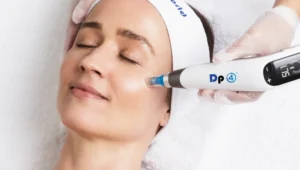 Dermal Pen Infusion Protocol
Dermal Pen Infusion Protocol
Using a dermal pen is one of the most effective ways to deliver exosomes deep into the skin:
- Skin Preparation – Cleanse thoroughly, apply antiseptic.
- Microneedling Session – Select needle depth (0.25–0.5 mm for infusion, 0.5–1.5 mm for remodeling) depending on indication.
- Exosome Application – Apply product during and immediately after Microneedling passes to maximize transdermal delivery.
- Post-Treatment – Apply calming mask or barrier serum; avoid occlusive heavy creams for 12–24 hours.
- Aftercare – Sun protection, gentle cleansing, no active exfoliants for 3–5 days.
- Final Thoughts
Exosomes represent a new era in regenerative aesthetics — offering a biologically intelligent way to restore youthful skin, enhance treatment results, and improve healing. When paired with precision delivery tools like the dermal pen, they can significantly boost patient outcomes.
As with any advanced treatment, practitioner training, product quality, and patient selection remain critical for safety and success.
For exosome application with a dermal pen, the most effective approach is during and immediately after Microneedling — not strictly “before” or “after” only
During and After Works Best
- Microneedling creates microchannels in the epidermis and superficial dermis.
- If exosomes are applied before starting, much of the product will sit on intact skin and not penetrate efficiently.
- If applied only after, microchannels may already be starting to close, reducing uptake.
- Applying during ensures the exosomes are driven into freshly created channels, and continuing application after maximizes saturation.
Why Applying During and After Microneedling Works Best
1. Microneedling Creates Transient Microchannels that Close Over Time
- Rapid skin resealing: Electrical impedance and transepidermal water loss (TEWL) studies show that the skin’s barrier typically begins recovering within 2–4 hours after Microneedling in non-occluded conditions PubMedPMC.
- Pore closure timeline: Visualization via calcein imaging reveals that closure completes around 12 hours post-treatment for shorter needles (~370 µm) and up to 18 hours for longer ones (~770 µm) PMCPubMed.
- Within 15 hours under ambient conditions: Other models show complete channel closure within ~15 hours, though occlusion (e.g., dressings) can extend this to up to 72 hours PubMedPMC.
Takeaway: There’s a finite window—especially the first few hours post-Microneedling—when the skin is most permeable and receptive to exosomal penetration.
2. Applying Exosomes Before Microneedling Isn’t Efficient
Applying exosomes before the Microneedling pass means most of the product remains on the intact stratum corneum and doesn’t reach deeper layers effectively. The initial needle passes may only sweep the topically applied product aside, rather than facilitating targeted delivery.
3. After-Only Application Risks Missed Delivery Opportunity
If exosomes are applied solely after Microneedling, microchannels may already be partially closed, reducing absorption—and thus potency. The greatest delivery occurs when the channels are freshly open.
4. During + After = Maximum Uptake & Retention
- During Microneedling: Applying exosomes in real time ensures they are directly driven into newly formed channels, maximizing initial delivery.
- After Microneedling: A final post-treatment application leverages still-open microchannels, supporting deeper and more complete penetration.
5. Clinical and Research Support
- Pilot study: Combining Microneedling with stem-cell derived exosomes showed significant improvements in pore size and texture—a synergy attributed to exosome delivery through microchannels PMC.
- Case study: Two sessions of superficial Microneedling with immediate exosome application yielded prolonged benefits (nearly two years!), including lasting pore refinement, reduced erythema, and improved pigmentation—highlighting the regenerative effects of well-timed delivery SpringerLink.
- Penetration limitations: Exosomes are large and complex molecules; less than 1% penetrate intact skin when applied topically, underscoring the need for Microneedling-assisted delivery Dr Rachel Ho.
Clinical Recommendations
- Apply a light layer of exosomes just before Microneedling—this can act as a glide serum and initiate penetration.
- Reapply exosomes during the session, section by section—ensuring fresh channels are saturated.
- Finish with a generous post-treatment application, massaging gently to maximize dermal absorption.
- Maintain product on the skin for at least several hours—or even overnight if product permits—for continued diffusion through residual open channels.
 At Vancoderm Academy, we are committed to equipping our students, alumni, and professional community with the latest evidence-based knowledge, advanced protocols, and hands-on training in cutting-edge therapies like exosomes.
At Vancoderm Academy, we are committed to equipping our students, alumni, and professional community with the latest evidence-based knowledge, advanced protocols, and hands-on training in cutting-edge therapies like exosomes.
Stay connected with us for more clinical insights, research updates, and advanced treatment techniques in medical aesthetics. Follow Vancoderm Academy on our official channels for articles, case studies, and upcoming professional workshops. Together, we can shape the future of aesthetic medicine.
🔗 Follow us on:
-
Instagram: @VancodermAcademy
-
LinkedIn: Vancoderm Academy
-
Facebook: Vancoderm Academy Official
Thank you for taking the time to read and learn with us. Your curiosity and commitment to professional growth are what drive innovation in our industry. We look forward to continuing this journey with you.
Thank you

