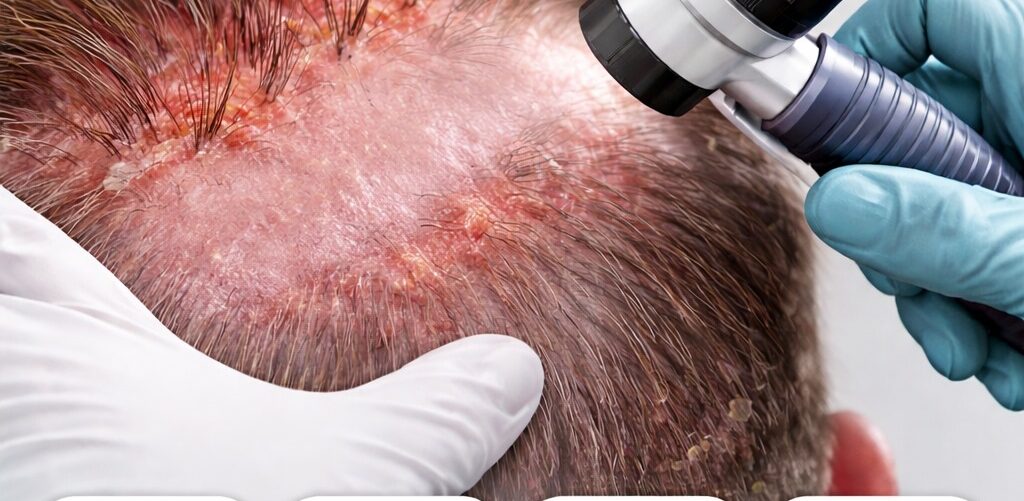
Folliculitis Decalvans
 Faramarz Rafie MD / Vancoderm Academy and College (VDA) / Vancoderm Clinic (VDCMED)
Faramarz Rafie MD / Vancoderm Academy and College (VDA) / Vancoderm Clinic (VDCMED)
Folliculitis Decalvans is a chronic neutrophilic inflammatory disorder targeting the hair follicles, characterized by progressive and permanent scarring alopecia due to follicular destruction and fibrosis. It predominantly affects the scalp but may also involve other hair-bearing regions. Folliculitis decalvans is classified within the spectrum of primary cicatricial (scarring) alopecias.
Epidemiology and Risk Factors:
- Age: Folliculitis decalvans most commonly presents in adulthood, typically during the fourth to fifth decades of life, although cases have been reported in younger and older individuals.
- Sex: There is a male predominance, with reported male-to-female ratios of approximately 3:1.
- Race and Skin Type: The condition occurs across all ethnicities. Some evidence suggests that disease severity, lesion distribution, and scarring patterns may vary in individuals with darker skin types.
- Risk Factors: The precise predisposing factors remain incompletely defined. Proposed contributors include host immune dysregulation, abnormal neutrophilic responses, genetic susceptibility, and bacterial colonization, particularly by Staphylococcus aureus. Environmental or mechanical triggers may exacerbate disease activity, but no single factor has been definitively established as causal.
Etiology
The precise etiology of folliculitis decalvans remains incompletely understood and is considered multifactorial. The most widely supported pathogenic hypothesis implicates a dysregulated host immune response to Staphylococcus aureus, a commensal bacterium of the skin and scalp. In affected individuals, S. aureus appears to act as a persistent inflammatory trigger rather than a primary infectious agent, leading to chronic follicular inflammation.
Current evidence suggests that abnormal innate immune activation, particularly involving neutrophils, results in exaggerated inflammatory signaling at the level of the hair follicle. This process promotes recurrent follicular pustulation, progressive follicular destruction, and eventual fibrotic replacement of pilosebaceous units. The inability of the host to effectively clear or tolerate bacterial antigens may contribute to ongoing disease activity.
Additional contributing factors proposed in the literature include:
-
Altered scalp microbiome, with increased S. aureus colonization and reduced microbial diversity
-
Defects in follicular immune privilege, rendering hair follicles more susceptible to immune-mediated injury
-
Genetic predisposition, supported by rare familial cases and associations with immune regulatory pathways
-
Abnormal neutrophil chemotaxis and cytokine release, perpetuating tissue damage
-
Environmental or mechanical triggers, such as scalp trauma, occlusion, or secondary infection, which may exacerbate disease onset or progression
Importantly, folliculitis Decalvans is not considered a simple infectious condition, as bacterial eradication alone does not consistently induce sustained remission. Rather, it represents a chronic inflammatory disorder with secondary bacterial involvement, where immune dysregulation plays a central pathogenic role.
Mechanism (Pathophysiology):
The pathophysiology of folliculitis Decalvans is characterized by a chronic, follicle-centered neutrophilic inflammatory cascade that culminates in irreversible follicular destruction and scarring alopecia. The disease process is initiated at the level of the pilosebaceous unit, with inflammation preferentially targeting the upper and mid portions of the hair follicle.
In the early stages, persistent follicular inflammation is frequently associated with colonization or overgrowth of Staphylococcus aureus. Rather than acting as a direct pathogen, S. aureus is believed to function as an immunologic trigger, stimulating an exaggerated innate immune response. This leads to excessive recruitment and activation of neutrophils, driven by pro-inflammatory cytokines and chemokines.
The resulting neutrophilic infiltration of the follicular epithelium causes follicular pustule formation, perifollicular erythema, crusting, and suppuration. Recurrent inflammatory episodes progressively damage the follicular wall, leading to rupture of the follicle and release of keratin, bacteria, and hair shaft fragments into the surrounding dermis. This amplifies the inflammatory response and extends tissue injury beyond the follicle itself.
As inflammation becomes chronic, there is progressive destruction of follicular stem cells located in the bulge region, which are essential for hair regeneration. Loss of these regenerative cells results in obliteration of follicular ostia, clinically manifesting as smooth, shiny areas of scarring alopecia with characteristic tufted hair formations at lesion margins.
Over time, the inflammatory infiltrate evolves from predominantly neutrophilic to a mixed inflammatory population including lymphocytes, plasma cells, and histiocytes. This transition is accompanied by fibroblast activation and collagen deposition, leading to permanent fibrosis and replacement of normal follicular architecture with scar tissue. Once fibrosis is established, hair regrowth is no longer possible.
Histopathologically:
-
Early lesions demonstrate dense neutrophilic infiltrates within and around the follicular epithelium, follicular pustules, and perifollicular inflammation.
-
Advanced lesions reveal follicular dropout, loss of sebaceous glands, perifollicular fibrosis, and mixed chronic inflammatory infiltrates consistent with cicatricial alopecia.
Diagnosis:
The diagnosis of folliculitis decalvans is primarily clinical, based on characteristic scalp findings and disease behavior, and is supported by trichoscopic, microbiological, and histopathological evaluations when the diagnosis is uncertain or when differentiation from other cicatricial alopecias is required.
A thorough medical history and physical examination are essential first steps. Clinicians assess the duration and progression of hair loss, presence of inflammatory symptoms such as pain, burning, or pruritus, prior treatments, and episodes of pustulation or secondary infection. On physical examination, the scalp typically reveals patchy scarring alopecia with active inflammatory borders, follicular pustules, crusting, erythema, and the hallmark finding of tufted hair follicles, in which multiple hair shafts emerge from a single dilated follicular opening.
Trichoscopy (Trichoscopy of the scalp) is a valuable noninvasive diagnostic tool that enhances diagnostic accuracy. Common trichoscopic features include:
-
Tufted hairs (polytrichia)
-
Perifollicular erythema and scaling
-
Follicular pustules and yellow crusts
-
Loss of follicular openings in scarred areas
These findings help distinguish folliculitis Decalvans from other inflammatory and scarring alopecias.
Microbiological assessment, including bacterial swabs from pustules or affected scalp areas, is frequently performed to detect Staphylococcus aureus. While bacterial colonization is not diagnostic in isolation, identifying S. aureus can guide antibiotic selection, monitor antimicrobial resistance, and support targeted therapeutic strategies.
A scalp skin biopsy is recommended in atypical presentations, early disease without classic features, or when alternative diagnoses are suspected. Histopathological examination confirms the presence of a neutrophilic cicatricial alopecia and excludes other entities such as lichen planopilaris, discoid lupus erythematosus, or fungal infections. Biopsy findings vary by disease stage, with early lesions showing neutrophil-predominant perifollicular inflammation and later lesions demonstrating follicular loss, mixed chronic inflammation, and fibrosis.
Signs and Symptoms
Typical features are:
- Patchy, progressive hair loss with scarring.
- Pustules, crusts and erythema, especially at lesion margins.
- Tufted hair appearance (“doll’s hair” clusters).
- Itch or burning pain may be present but variable.
- Scalp tightness or discomfort.
Differential Diagnosis:
The differential diagnosis of folliculitis decalvans (FD) encompasses a range of scarring (cicatricial) and inflammatory alopecias, as well as infectious and mechanical scalp disorders, given the overlap in clinical features such as pustules, erythema, crusting, and hair loss. Accurate differentiation is critical because management strategies and prognoses vary substantially.
Key conditions to consider include:
Tinea capitis (fungal scalp infection): Caused by dermatophytes, primarily Trichophyton species. Typically affects children, though adults may be involved. Clinical features include patchy hair loss, scaling, broken hairs, and occasionally pustules (kerion).
Diagnosis is supported by KOH examination, fungal culture, or PCR. Unlike FD, tinea capitis is reversible with antifungal therapy and does not usually cause permanent scarring in early stages.
Dissecting cellulitis of the scalp (perifolliculitis capitis abscedens et suffodiens):
Chronic, suppurative scarring alopecia predominantly in adult males. Characterized by deep nodules, sinus tract formation, and purulent drainage. Unlike FD, lesions are often coalescent, with extensive boggy plaques, and histology shows mixed neutrophilic and granulomatous infiltrates extending into the dermis.
Lichen planopilaris (LPP) / Frontal fibrosing alopecia (FFA):
Immune-mediated cicatricial alopecias marked by lymphocytic inflammation rather than neutrophilic predominance. LPP presents with perifollicular erythema, scaling, and progressive patchy alopecia; FFA is a variant with frontal hairline recession. Trichoscopy and biopsy demonstrate lichenoid interface dermatitis at the follicular epithelium, distinguishing them from FD.
Acne keloidalis nuchae (AKN): Chronic folliculitis affecting the nape of the neck, leading to papules, pustules, and eventual keloidal scarring. Typically occurs in men of African descent. Lesions are localized to the occipital and nuchal scalp, unlike FD which primarily affects vertex and mid-scalp.
Other primary cicatricial alopecias:
Includes central centrifugal cicatricial alopecia (CCCA) and other lymphocytic or mixed inflammatory scarring alopecias. Histopathology and distribution patterns aid differentiation from FD.
Diagnostic approach in ambiguous cases:
- Integration of clinical history, lesion morphology, and disease distribution is critical.
- Trichoscopy can highlight characteristic features (e.g., tufted hairs in FD).
- Scalp biopsy with histopathology remains the gold standard for differentiating cicatricial alopecias.
- Microbiological evaluation helps identify secondary infections, particularly S. aureus, which may exacerbate FD but is not a primary cause in other scarring alopecias.
Treatments of Folliculitis Decalvans
Folliculitis decalvans (FD) is a chronic, scarring alopecia for which there is currently no universally curative therapy. Management is primarily aimed at controlling inflammation, preventing disease progression, and minimizing new scarring. Treatment is guided by disease severity, activity, and patient-specific factors. Recent updates, including the EADV 2025 position statement and systematic reviews of therapeutic outcomes, emphasize a multimodal, individualized approach.
1. First-Line / Core Therapies (Antimicrobial Control)
-
Combination oral antibiotics: The rifampicin + clindamycin regimen is widely used for active disease, targeting Staphylococcus aureus colonization and reducing inflammatory triggers. Therapy typically extends for several weeks to months depending on clinical response.
-
Tetracyclines (e.g., doxycycline, minocycline): Serve both antimicrobial and anti-inflammatory roles, particularly for mild to moderate disease.
-
Topical antiseptics and antibiotics (e.g., chlorhexidine, fusidic acid): Used adjunctively to reduce bacterial load and prevent secondary infection, particularly in flare-prone areas.
2. Anti-Inflammatory Therapies
-
Systemic corticosteroids: Short courses may be employed for acute flares to rapidly suppress neutrophilic inflammation. Long-term use is limited due to systemic adverse effects.
-
Topical or intralesional corticosteroids: Useful for localized disease activity, reducing perifollicular erythema, pustulation, and symptomatic inflammation without systemic toxicity.
3. Retinoids
-
Oral isotretinoin: Indicated for mild, refractory, or relapsing disease. It modulates follicular keratinization and has anti-inflammatory effects, contributing to long-term disease control. Close monitoring is required for teratogenicity and laboratory changes (lipids, liver enzymes).
4. Advanced and Emerging Therapies
-
Biologic agents (e.g., TNF-alpha inhibitors such as adalimumab) and JAK inhibitors are under investigation for severe, treatment-resistant cases, targeting immune dysregulation driving chronic follicular inflammation.
-
Photodynamic therapy (PDT) and certain laser modalities have shown efficacy in select patients by reducing follicular bacterial load and local inflammation.
-
Experimental interventions such as platelet-rich plasma (PRP) or botulinum toxin injections have been reported in case studies but remain investigational.
5. Surgical Options
-
Surgical excision may be considered for localized, refractory lesions, particularly when medical therapy fails. Outcomes are generally favorable with low recurrence if disease is well-controlled preoperatively.
6. Hair Restoration
-
Hair transplantation can be considered in areas of stable, inactive disease once inflammation has been fully controlled. Pre-transplant evaluation is critical to prevent graft failure or disease reactivation.
Side Effects and Adverse Effects of Treatments
Treatment of folliculitis Decalvans is primarily aimed at controlling inflammation and preventing disease progression, as there is no therapy that can reverse established scarring. While effective in limiting follicular destruction, all interventions carry potential adverse effects that must be carefully considered and monitored.
1. Systemic Antibiotics
-
Potential adverse effects: Gastrointestinal disturbances (nausea, diarrhea, abdominal discomfort), hypersensitivity reactions, photosensitivity (tetracyclines), and disruption of normal microbiota.
-
Long-term considerations: Prolonged antibiotic use may contribute to antimicrobial resistance, including methicillin-resistant Staphylococcus aureus (MRSA). Monitoring and judicious use are recommended.
2. Systemic Corticosteroids
-
Common adverse effects: Weight gain, fluid retention, hyperglycemia, hypertension, mood changes, and immunosuppression.
-
Long-term risks: Osteoporosis, adrenal suppression, and increased susceptibility to infections; therefore, systemic steroids are generally reserved for short-term flare control.
3. Oral Retinoids (Isotretinoin)
-
Common adverse effects: Xerosis (dry skin, lips), cheilitis, mucosal dryness, and photosensitivity.
-
Serious considerations: Teratogenicity (strict contraception required), elevated serum lipids and liver enzymes; laboratory monitoring is mandatory.
4. Immunomodulators / Biologic Therapies (e.g., TNF-alpha inhibitors, JAK inhibitors)
-
Potential risks: Increased susceptibility to infections (bacterial, viral, opportunistic), infusion or injection-site reactions, and potential for systemic immunosuppression. Long-term safety data in FD remain limited.
5. Photodynamic Therapy (PDT)
-
Common adverse effects: Localized erythema, edema, burning sensation, and photosensitivity for several days post-treatment. Careful patient education on sun protection is essential.
6. Surgical Excision
-
Potential complications: Scarring, delayed wound healing, infection, and standard perioperative risks. Surgical intervention is typically reserved for localized, refractory lesions after disease control.
Clinical Note
- Importantly, none of these therapies restore hair once scarring has occurred, emphasizing the need for early diagnosis and timely intervention to prevent irreversible follicular loss.
- Adverse effects should be weighed against potential benefits, and patients require careful monitoring and counseling throughout treatment.
Latest Clinical Updates:
Recent clinical evidence has refined the therapeutic approach to folliculitis Decalvans (FD), emphasizing individualized, combination-based strategies to optimize long-term disease control and minimize scarring.
Systematic Review of Treatment Outcomes:
A 2025 systematic review analyzing 246 treatment modalities demonstrates that combination therapy, particularly systemic or topical antibiotics alongside anti-inflammatory agents, provides superior disease control and longer remission periods compared with antibiotic monotherapy.
This supports the concept that FD is not a purely infectious process; immune-mediated inflammation is a critical driver of follicular destruction. Consequently, interventions must target both microbial triggers and inflammatory pathways to achieve optimal outcomes.
EADV 2025 Position Statement and Therapeutic Algorithm:
The European Academy of Dermatology and Venereology (EADV) 2025 position statement provides a structured, evidence-based algorithm for FD management.
Key recommendations include:
Assessment of disease activity and severity to guide therapy intensity.
Stepwise use of combination systemic antibiotics (e.g., rifampicin + clindamycin) with adjunctive topical antiseptics or corticosteroids for active disease.
Early consideration of retinoids (isotretinoin) or immunomodulators for refractory or relapsing cases.
Use of advanced therapies—biologics, JAK inhibitors, photodynamic or laser therapy—reserved for severe, treatment-resistant disease.
The algorithm emphasizes tailored treatment regimens rather than a one-size-fits-all approach, highlighting the importance of early, aggressive control of inflammation to prevent irreversible scarring.
Clinical Implications:
- These updates reinforce that early recognition and multimodal therapy are essential in preventing disease progression.
- Long-term management should integrate antimicrobial control, anti-inflammatory therapy, and ongoing monitoring, with consideration of emerging therapies in refractory cases.
- The shift toward personalized treatment plans marks a significant advance in FD management, recognizing the interplay between microbial triggers and host immune dysregulation.
 Vancoderm Academy and College is recognized as a leader in medical aesthetics education in Canada, offering advanced training programs designed to develop highly skilled professionals. Our Clinical Practitioner Specialist Diploma in Medical Aesthetics is a comprehensive program that covers the full spectrum of aesthetic practice, including specialized trichology courses, providing students with in-depth knowledge and practical expertise in hair and scalp disorders. Vancoderm Academy also offers approved trichology certification programs, recognized by the BC Ministry of PSFS and the PTIRU organization, ensuring that graduates receive credentials that meet the highest professional standards in Canada.
Vancoderm Academy and College is recognized as a leader in medical aesthetics education in Canada, offering advanced training programs designed to develop highly skilled professionals. Our Clinical Practitioner Specialist Diploma in Medical Aesthetics is a comprehensive program that covers the full spectrum of aesthetic practice, including specialized trichology courses, providing students with in-depth knowledge and practical expertise in hair and scalp disorders. Vancoderm Academy also offers approved trichology certification programs, recognized by the BC Ministry of PSFS and the PTIRU organization, ensuring that graduates receive credentials that meet the highest professional standards in Canada.
We sincerely thank our readers for their continued support and invite you to follow us on LinkedIn, Instagram, and Facebook for updates, tips, and educational resources. Wishing you a Happy and prosperous New Year 2026!